Organisation Structure
Malaria Vaccine Pilot Evaluation-Case Control
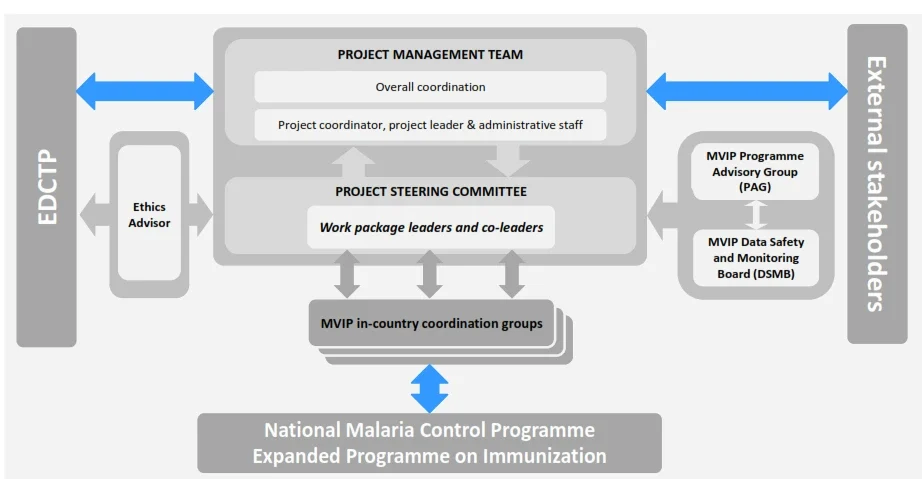
| Body | Function | Composition |
|---|---|---|
| Project management team | Coordinates and provides technical, managerial and administrative support to the project. Organise meetings and coordinates reporting activities | KHRC project coordinator, EVI project leader, KHRC project manager KHRC finance manager EVI project manager EVI finance manager |
| Project steering committee | Decision-making on design, project management, implementation of the project, consortium agreement and conflict resolution | Institutional representatives, coordinator EDCTP |
| Ethics Advisor | Advise on ethical issues, check for compliance with ethical standards and provide annual report Review consortium documents | Independent Ethics Advisor |
| MVIP Programme Advisory Group | Monitors programme activities and provides scientific, technical and programmatic advice to Programme Leadership Team No executive, regulatory or decision-making function. Reports to Strategic Advisory Group of Experts on immunization & Malaria Policy Advisory Committee (https://www.who.int/initiatives/malaria-vaccine-implementation-programme/programme-advisory-group) |
Nine experts in the fields of public health, epidemiology, malaria, vaccines, implementation research, statistics, immunization and policy. |
| MVIP Data Safety Monitoring Board (DSMB) | Review safety data from the MVPE and the GSK Phase IV study on an ongoing basis in order to monitor and rapidly identify any accumulating safety signals from across the programme. Provides updates to the Global Advisory Committee on Vaccine Safety Can recommend for the pilots to be stopped or altered in the event there is evidence of harm |
Six experts in public health, epidemiology, vaccine, malaria including cerebral malaria, meningitis, child health, implementation research, statistics |
| MVIP in- country coordination groups (National Malaria Control Programme (NMCP), Expanded Programme of Immunisation (EPI)) | Review safety data from the MVPE and the GSK Phase IV study on an ongoing basis in order to monitor and rapidly identify any accumulating safety signals from across the programme. Provides updates to the Global Advisory Committee on Vaccine Safety Can recommend for the pilots to be stopped or altered in the event there is evidence of harm |
Six experts in public health, epidemiology, vaccine, malaria including cerebral malaria, meningitis, child health, implementation research, statistics |
.png)